Functional Signaling and Cardiac Regeneration Group
Research Focus
The regenerative potential of the adult heart is very limited and insufficient to replace damaged muscle mass in the diseased heart. Recent advances in cardiac cell therapy and tissue engineering fuel new hope for the development of novel therapeutic approaches with the aim to trigger myocardial regeneration after injury. Stem cell-derived (iPSC-) cardiomyocytes represent ideal candidates for cardiac cell-based therapeutic strategies, and current research focuses on the development of cardiac constructs for implantation. Despite the cardiogenic properties of the newly generated cardiomyocytes, these cells present a heterogeneous and immature phenotype, which is more comparable with cardiomyocytes of early developmental stages. However, successful employment of these new cardiomyocytes for myocardial repair demands that the physiological profile of iPSC-cardiomyocytes matches with the functional complexity of mature cardiomyocytes. This is currently not the case. We are interested in gaining better insight into the mechanisms that drive cardiac maturation and follow different approaches to enhance the cardiac phenotype of iPSC-cardiomyocytes.
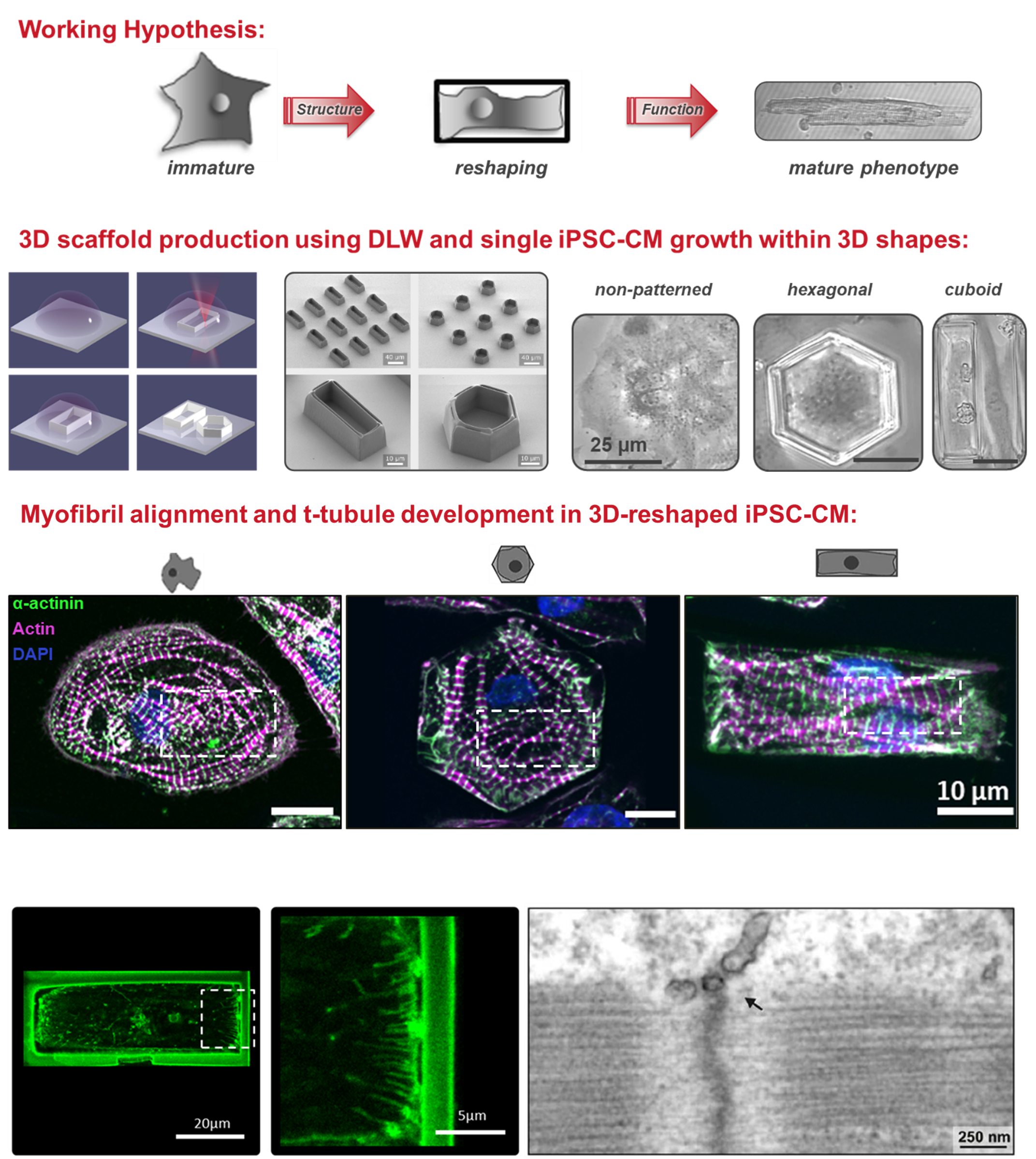
Project 1: Shaping the Heart – Structural and Functional Characterization of Human iPSC-Cardiomyocytes
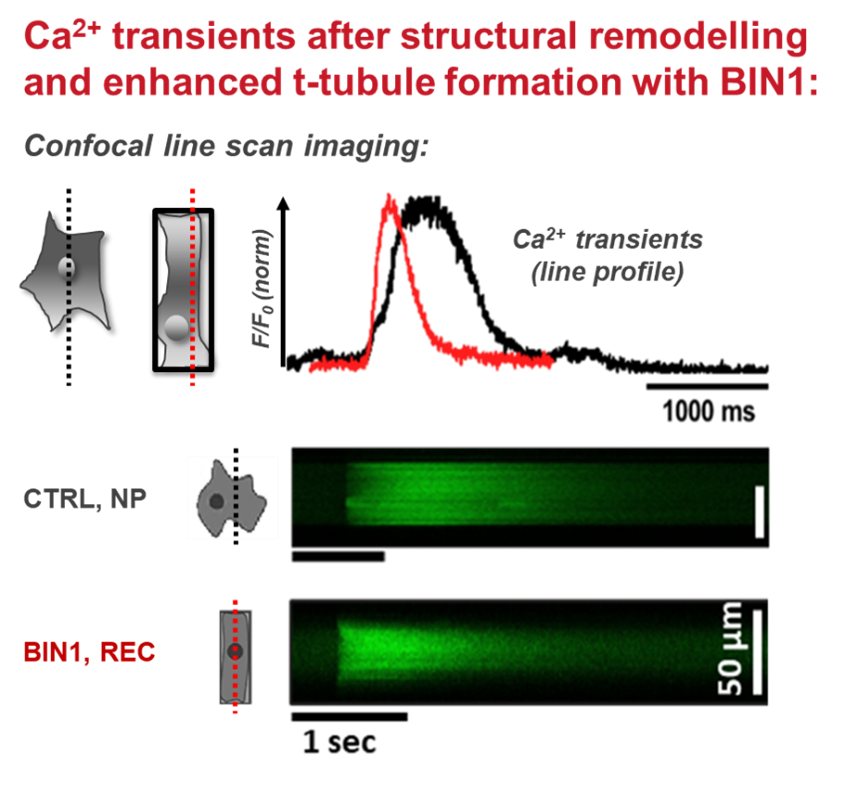
The adult mammalian cardiomyocyte is a highly specialized and terminally differentiated cell with distinct structural features. A precise microarchitecture, e.g. a regular transverse (t)-tubular network and strict myofilament organization, is a prerequisite for the sophisticated functional specialization of heart cells and provides the basis for local calcium signaling domains and protein interaction clusters for efficient excitation-contraction (EC) coupling. Lack of this microarchitecture in premature iPSC-cardiomyocytes strongly limits the efficiency of cell contraction and force production. These deficits may be overcome by triggering cellular maturation through optimized in vitro culture conditions, aiming at better mirroring the in vivo situation. In this project, we test the hypothesis that a specific cell geometry influences the subcellular microarchitecture of iPSC-cardiomyocytes with the aim to trigger morphological and functional maturation in these cells and to approach a similar phenotype as mature myocytes. In a multi-disciplinary approach combining the expertise in material science and bio-engineering with cardiac cell physiology, we analyze the effects of material contact and specific predefined geometries on cell shape and function at the level of single cells. Furthermore, outside-in signaling cascades and specific proteins regulating sarcolemmal properties are investigated under different experimental conditions.
Funding
This project has been started and funded by the SNF-Ambizione Fellowship (2011-2014) with continuous funding by the research bridge «Synthetic Biology» established by HEiKA as a collaboration with the lab of Prof. Dr. Martin Bastmeyer at the KIT (2014/2015), and by the German Research Foundation (DFG, 2020-2023).
Selected Publications
Shaping the heart: Structural and functional maturation of iPSC-cardiomyocytes in 3D-micro-scaffolds. Biomaterials 227:119551, 2020 DOI
Substrate Stiffness Influences Structural and Functional Remodeling in Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Front. Physiol 12:710619, 2021 DOI
Membrane remodelling triggers maturation of excitation-contraction coupling in 3D-shaped human-induced pluripotent stem cell-derived cardiomyocytes. Basic Res Cardiol 118(1):13, 2023 DOI
Project 2: Intercellular Communication in human iPSC-Cardiomyocytes
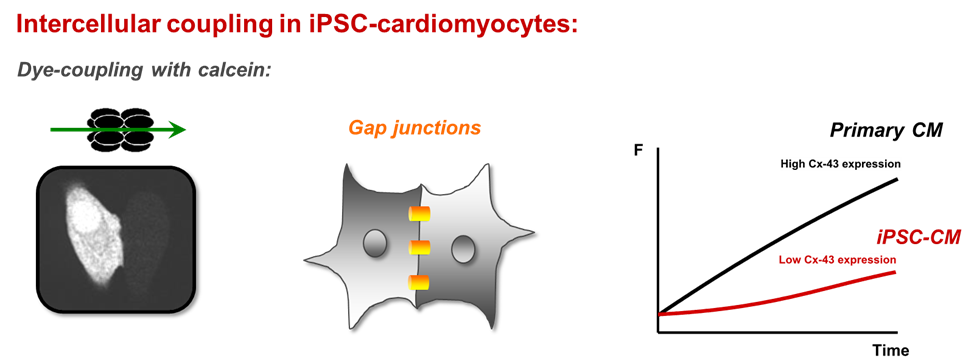
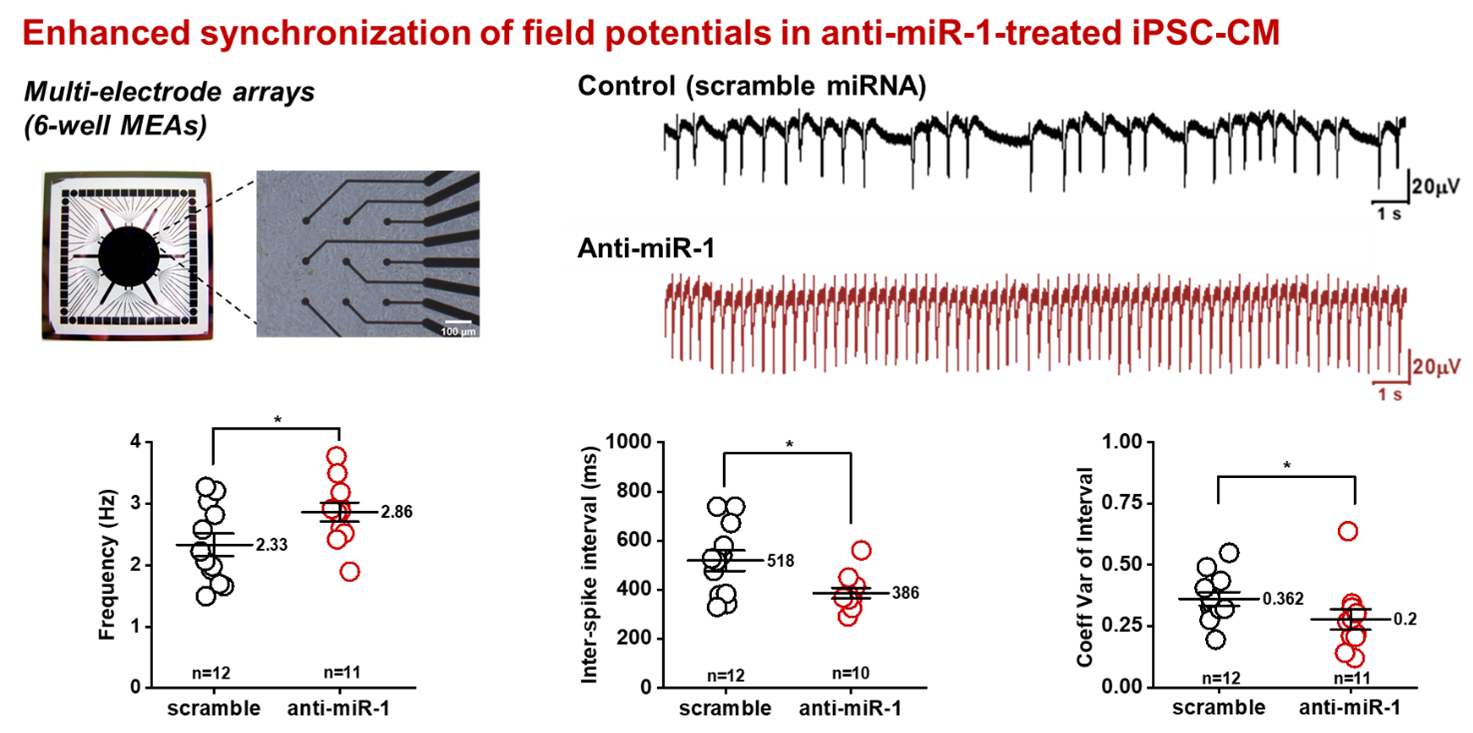
In the light of novel treatment options for ischemic cardiomyopathies and arrhythmogenic diseases, the functional properties of newly developed cardiomyocytes derived from iPS-cells are investigated and compared with primary cardiomyocytes at the cellular level and in multicellular preparations. One critical shortcoming of these novel cardiomyocytes is their limited potential to connect with native cardiomyocytes in order to establish a functional syncytium with similar electrophysiological properties. We have recently shown that in multicellular preparations cell-to-cell coupling is strongly compromised. Reduced coupling results in a strong reduction of the electrical signal transmission and conduction velocity, which may increase the risk for the development of arrhythmias in clinical settings. In this project, we are interested in deciphering the endogenous mechanisms that control cell excitability and intercellular communication by investigating the voltage-dependent Na channel, Cx43 expression and gap junction formation. The aim of this project is to enhance electrical signal transmission in hiPSC-cardiomyocytes and to improve heterocellular coupling with native cardiomyocytes to make these novel cardiomyocytes fit for clinical applications.
Funding
This project has been started and funded by the SNF-Ambizione Fellowship (2011-2014) with continuous funding by the Frontiers Excellence Cluster of the University of Heidelberg (2016-2018), the Elisabeth and Rudolf Hirsch Stiftung (2018), the CSC scholarship (2018-2020) and the DFG (2020-2023).
Selected Publications
Functional Characterization and Comparison of Intercellular Communication in Stem Cell-Derived Cardiomyocytes. Stem Cells 33(7):2208-2218, 2015 DOI
Slow conduction in mixed cultured strands of primary ventricular cells and stem cell-derived cardiomyocytes. Front. CellDev.Biol. 3:58, 2015 DOI
Improving electrical properties of iPSC-cardiomyocytes by enhancing Cx43 expression. J. Mol. Cell. Cardiol. 120:31-41, 2018 DOI
Distress-Mediated Remodeling of Cardiac Connexin-43 in a Novel Cell Model for Arrhythmogenic Heart Diseases. Int J Mol Sci 23(17):10174, 2022 DOI
